Establishing a Center of Excellence for Antimicrobial Susceptibility Testing
Context
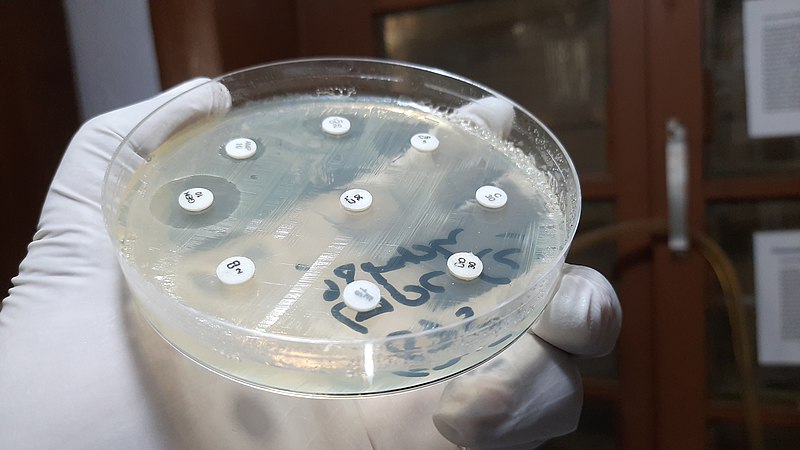
Antimicrobial Susceptibility Testing (AST) of microorganisms is essential for determining which antimicrobials could be effective treatment options in human and veterinary medicine. It is essential both for targeted treatment as well as for empirical treatment. Reliable AST capacities are also necessary for local, national and regional authorities to understand the circulating resistance levels and patterns in a particular district, county, or country and is therefore critical for good antimicrobial stewardship.
AST can be done either by phenotypic methods or predicted from genotypic data. AST results are translated into categories of the expected effect of a given antibiotic for a particular microorganism as susceptible, intermediate or resistant (S/I/R) or susceptible and non-susceptible (S/NS) by means of breakpoints.
Problem
Reliable AST is a prerequisite for the appropriate treatment of infections in humans, livestock and aquaculture. This is increasingly true with the increased antimicrobial resistance (AMR). Furthermore, surveillance of AMR development requires good quality data. However, continuous delivery of accurate AST results has proven difficult for routine laboratories all over the world. Without appropriate training, experience and proper equipment, there will be inconsistencies in interpretation of AST results, even when reliable and standardised methods are used. A range of questions regarding testing and interpretation of test results frequently arise. There is need for continuous training and hence a training center to expand expertise in AST as well as to provide much-needed support to laboratories when discrepancies or challenges arise.
“AMR is a major One Health challenge that could threaten millions of lives and livelihoods worldwide and this unique partnership will contribute to preventing the threat of AMR through capacity development and solutions tailored to LMICs.”
Jimmy Smith, Director General, ILRI (taken from original press release)
Project overview
This project will establish an AST Center of Excellence for serving countries in Africa, covering pathogens in humans, livestock and aquaculture. The Center will support both human and veterinary clinical microbiology laboratories in Africa in all aspects of AST, including the development of standard operating procedures, offer training, provide support for troubleshooting, and perform comparison and quality control across manufacturers.
The Center will also contribute to breakpoint setting by providing MIC distributions for antimicrobial-microorganism combinations with a focus on pathogens from humans, livestock and aquaculture. A skilled AST Center in Africa would fill a gap in the region, provide training and advice for laboratories in LMICs, and support quality-assured AST across One Health projects. This would also increase the ability to produce robust AST data that can be compared with that of other laboratories and submitted to global surveillance programmes.
It is anticipated that the Center will become a regional reference laboratory and training center for LMICs in Africa, with the capacity to conduct regular trainings on AST to technicians from other laboratories, and to provide technical support for troubleshooting on an ongoing basis. The Centre will be placed within the state of the art laboratory facilities at the International Livestock Research Institute (ILRI), in Nairobi, Kenya, which hosts the CGIAR Antimicrobial Resistance Hub and also hosts a Capacity Development Unit.
“The centre will be an important contribution to better AST in Sub-Saharan Africa – offering both capacity training, expansion on drug-bug MIC and disk diffusion data and evaluation of methods in collaboration with the EUCAST development laboratory.”
Robert Skov, Scientific Director, ICARS
Outcomes
- The development of a regional reference laboratory with evidence-based competence in AST methodology
- The development of a reference AST strain collection
- Several open workshops on AST methodology, with 10-20 participants from several African laboratories
- Development of a standardised curriculum
- A capacity of 3-5000 diagnostic/project samples processed per year
Results
Facts
Region: Africa
Sector: Humans
Country: Kenya
Type: Supporting activity
Country partners: International Livestock Research Institute (ILRI); European Committee on Antimicrobial Susceptibility Testing (EUCAST) Development Laboratory, Denmark
ICARS funding: 168, 586 USD
ICARS Science Team

Resources
Share
Share this project on socials